|
SODIUM METHOXIDE
| ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. |
124-41-4 |
|
| EINECS NO. | 204-699-5 | |
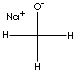
| FORMULA | CH3ONa | |
| MOL WT. | 54.02 | |
| H.S. CODE | 2905.19 | |
|
TOXICITY |
Oral rat LD50: 2037 mg/kg | |
| SYNONYMS | Sodium Methylate; Methanol, Sodium Salt; | |
| Sodium methanolate; Natriummethanolat (German); Metanolato de sodio (Spanish); Méthanolate de sodium (French); Metilato di sodio; Metanolato di sodio (Italian); | ||
|
SMILES |
sodium, methanol | |
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES (ANHYDROUS POWDER) |
||
| PHYSICAL STATE |
white powder | |
| MELTING POINT | 127 C (Decomposes) | |
| BOILING POINT |
| |
| SPECIFIC GRAVITY | 1.1 | |
| SOLUBILITY IN WATER | Reacts violently (miscible with methanol and ethanol) | |
| pH |
| |
| VAPOR DENSITY | ||
| AUTOIGNITION |
240 C | |
| NFPA RATINGS | Health: 3; Flammability: 2; Reactivity: 2 | |
|
REFRACTIVE INDEX |
| |
| FLASH POINT |
32 C | |
| STABILITY | Stable underordinary conditions. Hygroscopic. | |
|
GENERAL DESCRIPTION & APPLICATIONS |
||
|
Alkoxide (also called alcoholate ) is the conjugate bases of corresponding alcohol. They contain negatively charged oxygen atom, found as intermediaries in various reactions. Alkoxide is a strong reducing agent. The term alkoxide is for a compound formed from alcohol by replacing the hydrogen of the hydroxy group by a monovalent metal. Alkoxides provide carbanion in the relevant alcohols. A carbanion is an anion which arise cleavage of a covalent bond involving carbon and bears a negative charge. A carbanion is an unstable intermediate stage during a chemical reaction. Thus, it is encountered in organic synthesis. Metal alkoxides are versatile reagents favoring the chemical reaction of condensation, esterification, alkoxylation and etherification, Claisen condensation, Wolf-Kishner reduction and Stobbe reaction are examples. They are used in wide range of applications in organic synthesis; Agrochemicals; Pharmaceuticals, colorants and aroma chemicals. They are used in manufacturing detergents and biodiesel. They also act as catalysts in polymerization and isomerizations. Biodiesel is biodegradable, non-toxic fuel made from biolipids such as vegetable oils or animal fats as an candidate to replace fossil fuels. It should significantly reduce emissions when burned. Mostly biodiesel is produced using base catalyzed transesterification which exchanges the alkoxy group of an ester compound by another alcohol in the presence of acid or base catalyst. The transesterification process in biodiesel production is the reaction of a triglyceride with a bioalcohol to form esters (a biodiesel) and glycerol (a by-product). The most common form of biodiesel is methyl esters of long chain fatty acids , though ethyl ester biodiesel exits. Acids can catalyse the reaction by donating a proton to the alkoxy group, while bases can catalyse the reaction by removing a proton from the alcohol. Traditionally transesterification is for the production of polyester fiber. Diesters (e.g dimethyl terephthalate) undergo transesterification with diol (e.g ethylene glycol) to form macromolecule (polyethylene terephthalate) and methanol. The reverse reaction (methanolysis) is also transesterification, and has been used to recycle polyesters. Sodium methoxide is a very powerful base that is used as a base catalyst in the production of biodiesel. Potassium methoxide find similar applications. Higher quality of by-product (glycerol) is expected by using potassium methylate catalyst. |
||
| SALES SPECIFICATION | ||
| 30% IN METHANOL |
||
| APPEARANCE |
clear liquid | |
| CONTENT (CH3ONa) |
30.0% min | |
| WATER |
0.2% max | |
| NaOH |
0.3% max | |
| Na2CO3 |
0.3% max | |
| ANHYDROUS POWDER | ||
|
APPEARANCE |
white powder | |
|
ASSAY |
98.5.0% min | |
| NaOH + Na2CO3 |
1.5% max | |
| TRANSPORTATION | ||
| PACKING | 170kgs in drum (Solution) | |
| HAZARD CLASS | 4.2 (Packing Group: II) | |
| UN NO. | 1431 (powder), 1289 (solution) | |
| OTHER INFORMATION | ||
|
Hazard
Symbols: F C, Risk Phrases: 11-14-34, Safety Phrases: 8-16-26-43
|
||